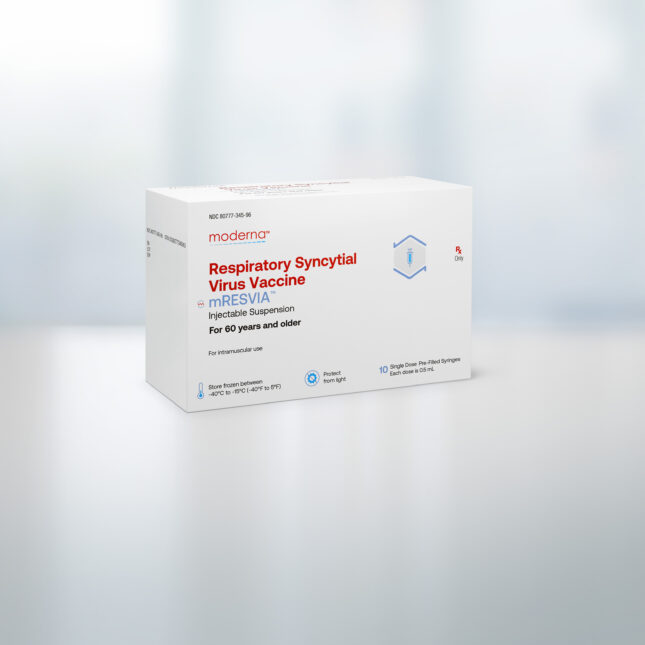
The Food and Drug Administration on Friday approved Moderna’s vaccine to protect older adults against respiratory syncytial virus, or RSV, making this the company’s second licensed product.
The Moderna RSV vaccine, which will be marketed under the name mResvia, was approved for adults 60 and older. It is made using the same messenger RNA platform as Moderna’s other licensed product, its Covid-19 vaccine Spikevax.
“The FDA approval of our second product, mResvia, builds on the strength and versatility of our mRNA platform,” Moderna CEO Stéphane Bancel said in a statement.
This article is exclusive to STAT+ subscribers
Unlock this article — plus daily coverage and analysis of the biotech sector — by subscribing to STAT+.
Already have an account? Log in
Already have an account? Log in
To submit a correction request, please visit our Contact Us page.










STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect