
NEW YORK — By the time Peter Bauman considered deep brain stimulation, he was desperate. Early onset Parkinson’s disease, diagnosed at age 49, had disabled him, ended his bartending career, and led him to consider suicide.
He hoped that the treatment, known as DBS, in which an electrode connected to an external battery is inserted into the brain and emits electrical impulses, would ease his Parkinson’s tremors.
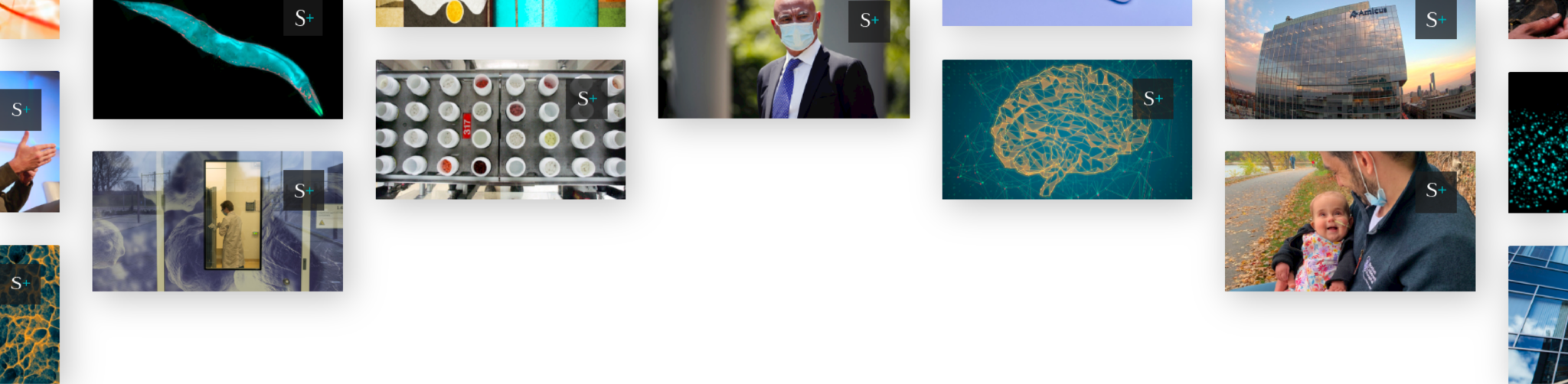
In March 2020, as Bauman was preparing to undergo the surgery at a Mount Sinai medical facility in midtown Manhattan, he said he was invited to participate in a research study — one only open to patients already committed to undergo DBS at Mount Sinai. Over the course of two DBS procedures, a neurosurgeon would take up to a 1-cubic-centimeter piece of tissue from both the left and right sides of the brain, to use for research. In study documents, Mount Sinai doctors said the biopsies result in “the same amount of tissue loss” and “in effect, the same level of risk” for patients as standard DBS, because they are removing tissue that would otherwise be cauterized.
Referred to at Mount Sinai as the Living Brain Project (LBP), the research aims to be the largest-ever molecular study of the living human brain, according to a National Institutes of Health grant application. While most research on the human brain is conducted on postmortem tissue, the researchers behind the LBP say the study of living tissue can help revolutionize our understanding of human brain biology.
Even though Bauman, now 58, was free to decline the research study and biopsies and still undergo DBS, he said he didn’t think twice. “I signed off on letting them take a little piece of my brain,” he recalled. “I just [was] kind of willing to sign anything to expedite the process, so I did.” At that point, he was living in a nursing home and had debilitating symptoms: tremors, difficulty gripping with his hands, and trouble walking. The brain biopsies seemed inconsequential. “I don’t remember them mentioning anything that it would hinder or harm.”
What Bauman couldn’t have known was that roughly one year earlier, the Mount Sinai brain biopsies had set off alarm bells at the Food and Drug Administration, whose review had been triggered when a device manufacturer wrote to the agency in April 2019. The manufacturer requested approval to modify an electrode Mount Sinai was using in its Living Brain Project, in order to briefly record certain brain activity. In late 2013, the FDA had conditionally approved the biopsies as a part of an early feasibility study, which it limited to six patients at the medical center who were getting DBS for treatment-resistant depression.
The FDA’s months-long internal examination of the LBP yielded a series of documents seen by STAT, including a lengthy review, completed in August 2019, of the Mount Sinai study. The review, which lists consultations with 16 different FDA officials, was scathing. It determined that the Mount Sinai doctors had been using a “false justification” to obtain patient consent to take the biopsies. One neurosurgeon consulted by the agency said any such biopsy “introduces serious risks to the human subjects.”
This article is exclusive to STAT+ subscribers
Unlock this article — plus in-depth analysis, newsletters, premium events, and networking platform access.
Already have an account? Log in
Already have an account? Log in
To submit a correction request, please visit our Contact Us page.





STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect